Medical Device Innovation Acceleration
Accelerated Digital Manufacturing Built for Class I, II, & III Medical Devices
DI Labs enables fast, safe, and compliant development through on-demand digital manufacturing. Our platform empowers teams to move from concept to clinical trials and commercialization – without tooling, without compromise.

Use Cases for Digital Manufacturing Through the Development Cycle
1. Concept & Strategy
- Concept mock ups
- Early prototypes
- Show & tell models
- Functional prototypes
- Idea validation
- Design of experiment models
- Functional prototypes
- Design validation
- DOE models
- Benchtop models
- Repeatable production
- Human factors optimization models
- On-demand production
- Responsive design updates
- Regulatory-ready documentation
- Bridge production
- Permanent low-volume production
- Post-launch design improvements (Class I Devices)
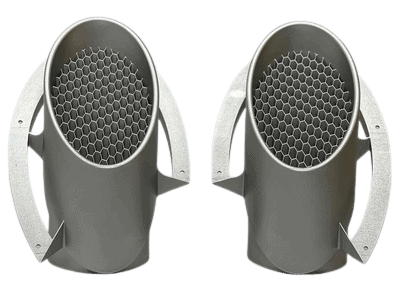
Example Housing & Assembly:

Supported Applications:
- Minimally Invasive Surgical Devices
- Cardiovascular Delivery and Retrieval Systems
- Medical Robotic Components
- Medical Device Housings & Assemblies
- Robotic End Effectors
Client Testimonials:
Traditional Pain vs Accelerated Digital Solutions

Expensive, design-locked tooling
An injection mold alternative with no expensive tooling and unlimited iteration freedom

Long lead times for small batches

On-demand, agile production & rapid responsiveness for clinical trial builds

Intense regulatory requirements

Regulatory compliance with ISO 10993 biocompatible materials, locked processes and lot-level power-to-part tracking

Design iteration is slow and expensive

Unlocked iteration for rapid integration of clinical feedback, interface optimization & efficacy improvements
Biocompatible Materials
| Title | Type of Material | Stiffness | Ultimate Strength | High Temperature Capability | Ductility | Impact Resistance | Gallery | Technical Document | |
|---|---|---|---|---|---|---|---|---|---|
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View | ||||||||
| Polymer | View |

Download |
|||||||
| Metal | View | ||||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
Learn More:
Reach out to us for support or more information!
[email protected] | 320-403-9483
"*" indicates required fields