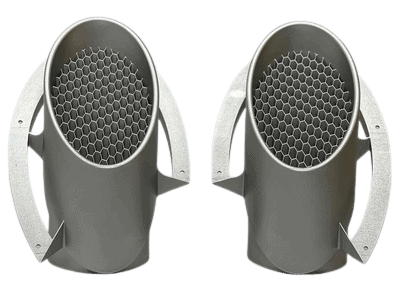
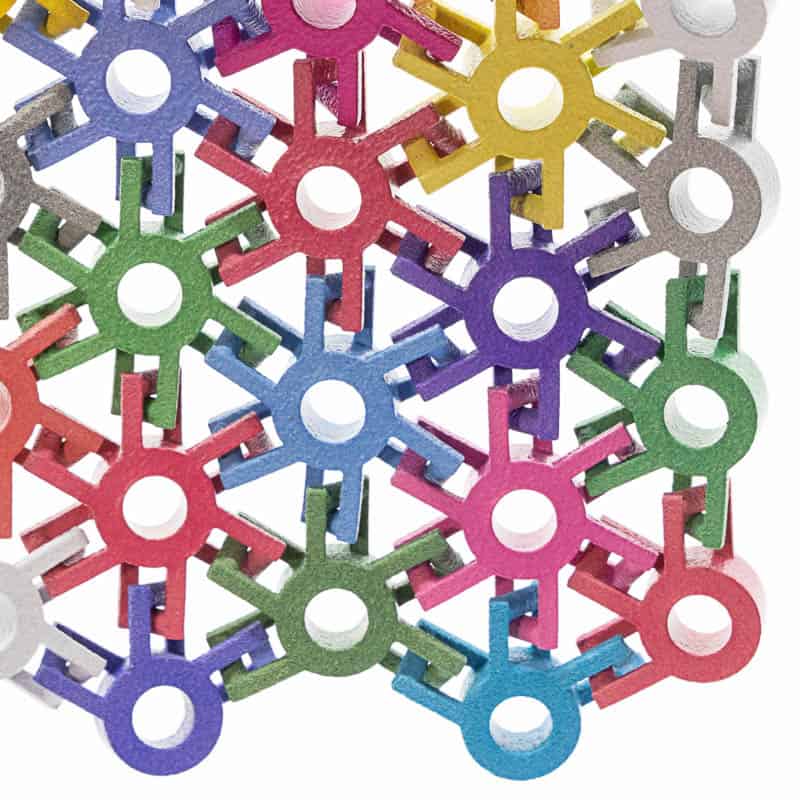
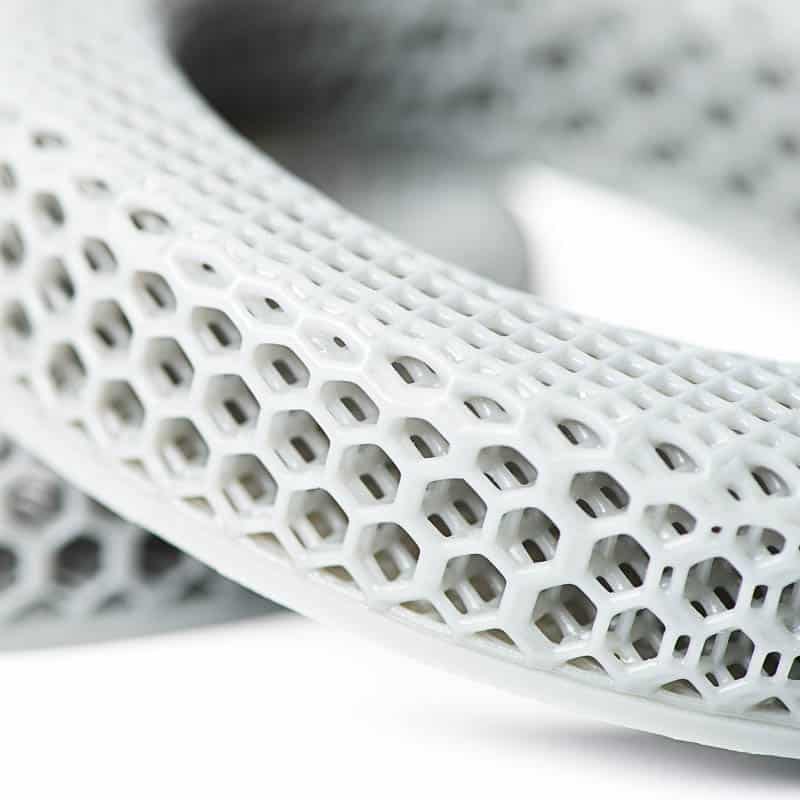
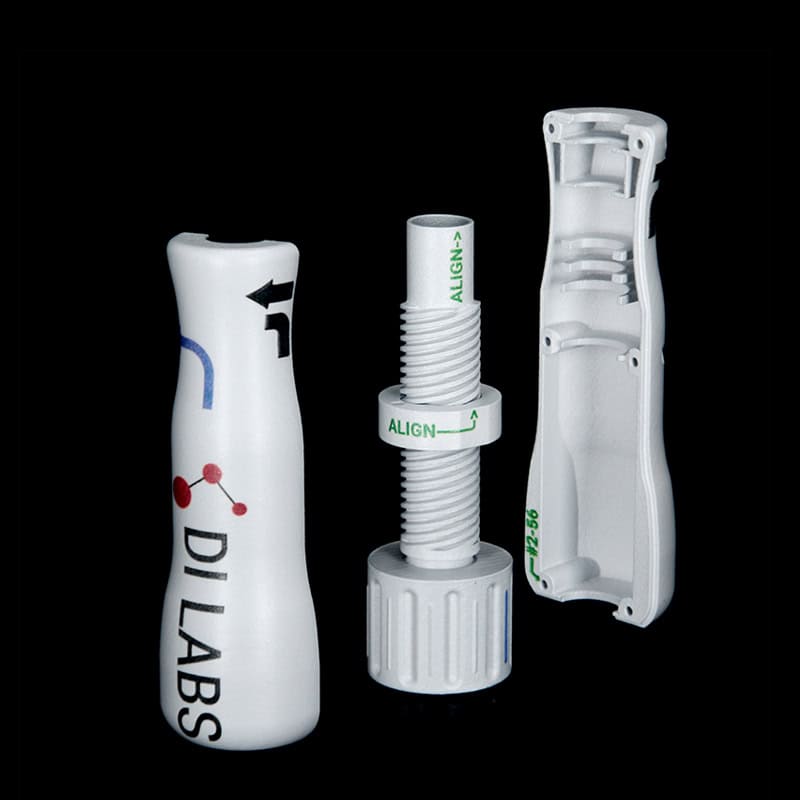
Class I, II & III Medical Device Manufacturing
An Injection Mold Alternative for Medical Device Manufacturing
DI Labs enables accelerated, compliant medical device development from concept to commercialization using digital manufacturing. Our advanced additive manufacturing technologies and highly controlled digital workflows empower teams to move quickly and efficiently through each development phase with unlimited design iteration and injection-mold-quality parts from concept to commercialization.
Example Medical Device Applications:
- Minimally Invasive Surgical Devices
- Cardiovascular Delivery and Retrieval Systems
- Medical Robotic Components
- Medical Device Housings & Assemblies
- Robotic End Effectors
DI Labs Additive Manufacturing vs Injection Molding
Biocompatible
Materials
DI Labs
Wide variety of biocompatible materials including polymers, elastomers & metals
Injection Molding
Wide variety of biocompatible materials including polymers, elastomers & metals
Tool-Free
Manufacturing
DI Labs
No tooling required, eliminating capital expense & long wait times
Injection Molding
Tooling required with significant capital expense & long wait times
On-Demand Batch Production
DI Labs
Short lead times to support acceleration through testing & clinical trials
Injection Molding
Long lead times for mold changes through testing & trials
Unlimited
Design Iteration
DI Labs
Tool-free enables responsive design iteration based on user feedback
Injection Molding
Expensive, design-locked tooling limits responsiveness & abilty to iterate

Our Biocompatible Materials
We offer a wide range of biocompatible materials – including polymers, elastomers, and metals – to serve a full spectrum of Class I, II, & III medical device applications. See our biocompatible materials table for more details.
Medical Device Housing & Assembly: Benefits of Additive Manufacturing

Use Cases for Additive Manufacturing Through the Medical Device Development Cycle
Concept & Strategy
- Concept mock ups
- Early prototypes
- Show & tell models
- Functional prototypes
- Idea validation
- Design of experiment models
- Functional prototypes
- Design validation
- DOE models
- Benchtop models
- Repeatable production
- Human factors optimization models
- On-demand production
- Responsive design updates
- Regulatory-ready documentation
- Bridge production
- Permanent low-volume prod.
- Post-launch improvements (Class I Devices)
Biocompatible Materials Table
| Title | Type of Material | Stiffness | Ultimate Strength | High Temperature Capability | Ductility | Impact Resistance | Gallery | Technical Document | |
|---|---|---|---|---|---|---|---|---|---|
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View | ||||||||
| Polymer | View |

Download |
|||||||
| Metal | View | ||||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |

What Medical Device Teams Really Want to Know About Additive
The questions following DI Labs’ technical session at MD&M West 2026 reflected a noticeable shift in how medical device teams are thinking about additive manufacturing. DI Labs CEO Carl Douglass delivered the session, which provided a deep dive into the realities of using additive manufacturing within regulated medical device programs. See the detailed Q&A in our LinkedIn newsletter.
Solutionology Stories Podcast Episodes From DI Labs
Example Medical Device Projects



Reach out for support or more information!
[email protected] | 320-403-9483
"*" indicates required fields
DI Labs Client Testimonials
Contact Us Directly