Medical Device Innovation Acceleration
Medical Device Innovation Accelerators for Class I, II, & III Applications
DI Labs offers digital manufacturing services to medical device developers and medical device contract manufacturers to accelerate their innovation process with speed, design agility and real-time iteration through clinical trials. We specializes in digitally manufactured components used in medical device assemblies, but our diverse fleet of technologies can support every stage from concept prototypes to pre-market validation and full-scale commercialization. DI Labs was founded by a team of product development and manufacturing engineers, giving us a unique understanding of the challenges and opportunities our clients face.
Supported Applications:
- Minimally Invasive Surgical Devices
- Cardiovascular Delivery and Retrieval Systems
- Medical Robotic Components
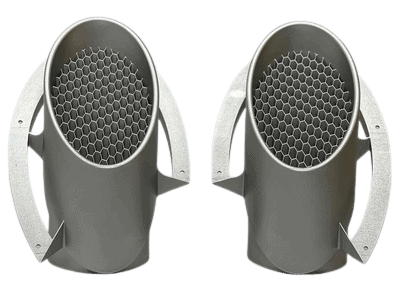
- Medical Device Housings & Assemblies
- Robotic End Effectors
Advantages Provided:
- Biocompatible components in days
- Accelerated design iteration during clinical trials
- Patient-safe prototypes
- Incorporating realtime physician feedback
- Limitation-free design opportunities
- DFAM assessment and guidance
- Opportunities for customization
Example Housing & Assembly:

How We Accelerate Innovation in the Stages of Medical Device Development:
- Vastly accelerated idea iteration & prototyping
- Enhanced tools for stakeholder communication
- Limitation-free design opportunities for market differentiation or customization
- Diverse offering of biocompatible materials
- Patient-safe, biocompatible prototypes in days
- Accelerated testing to validate usability, ergonomics & functionality
- Design freedom to create built-in features to support risk mitigation
- Vastly accelerated iteration cycles - hours or days vs. weeks or months
- Design freedom to reduce part count or assembly complexity
- Design freedom to build in visible guides to support HRT strategies
- More effective testing with functional prototypes at end-use quality
- Ability to incorporate realtime physician feedback & build their confidence
- Anatomical accuracy when printed from CT or MRI
- Design verification through mock-use scenarios using printed jigs & fixtures
- Vastly accelerated design iteration opportunities
- Possibility of patient-specific anatomical solutions
- Quick & cost-effective trial-specific guides, packaging or instruments
- Cross-sectional or exploded views for clinician training or submission visuals
- Accelerated low volume production for early-market use while tooling is finalized
- Tool-less production for low-to-mid volume or personalized devices
- Quick response to design updates and small batch production for inventory gaps
Biocompatible Materials
| Title | Type of Material | Stiffness | Ultimate Strength | High Temperature Capability | Ductility | Impact Resistance | Gallery | Technical Document | |
|---|---|---|---|---|---|---|---|---|---|
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View | ||||||||
| Polymer | View |

Download |
|||||||
| Metal | View | ||||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Elastomer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
|||||||
| Polymer | View |

Download |
Client Testimonials:
Learn More:
Please contact me. I'm interested in learning how DI Labs can help my company.
"*" indicates required fields